
(Photo of ice cubes from Habimama)
INVENTION
A lot of people say that one guy, Dr. John Gorrie, invented the ice cube. But actually, he invented refrigeration and an early version of air conditioner.
Gorrie believed that diseases like yellow fever wouldn't survive in cooler air (actually air temperature had nothing to do with it), so he designed a machine that would cool the air. The machine used compressed air to generate ice bricks, and the air flowing over the ice bricks was released into the room to cool it.

A model of the ice-making refrigeration machine that Dr. Gorrie invented. The ice bricks collected in the wooden box at the top, and the air flowed over the box.
(Photo from Gorrie's Fridge)
By my reckoning, the first guy to invent ice cubes as we know them today -- little hunks of ice used for the purpose of cooling beverages or food -- was an inventor named Lloyd Groff Copeman.
(Click the Read More link for the full entry)
Copeman invented all kinds of things -- a thermostat, an electric stove, a machine to turn toast so it would toast evenly on both sides, latches and switches for refrigerators, a machine that would automatically grease ball bearings on automobiles, all sorts of things.

This is Lloyd.
(Photo from Lloyd Copeman.com)
He was from a small town near Flint, Michigan, and he noticed one winter day that the icy slush that had collected on his rubber boots cracked and broke off very easily when he flexed the boot. This led him to invent the flexible rubber ice cube tray. This was in 1928.

Instructions for how to use Lloyd Copeman's flexible rubber ice cube tray
(Photo from Lloyd Copeman.com)
How we got from the flexible, easy-to-use rubber trays to those metal ice cube trays with the handle you had to yank up, I have no idea.

(Photo from icecubetray.org)
Did you have these in your house? We did when I was a kid, and they were so hard to use. The metal handle is connected to all the metal separators and when you pull up the handle, that's supposed to make the separators slant forward and thus crack and loosen the ice. But you had to yank up that handle really hard and even then sometimes the separators wouldn't move enough to loosen the ice and you'd have to work the handle up and down several times to loosen the ice. Sometimes there'd still be ice stuck to the separators or the tray itself, and ogh, those metal trays were a royal pain. If your hands were wet or even damp, your skin would stick-freeze to the metal. When my parents got a new refrigerator, it came with plastic ice cube trays, and those things were like a godsend to me as a child. So simple! So easy! I'm astounded to learn that the flexible trays were actually invented before the metal ones.
I think the metal ice cube trays are starting to get popular again, though, because people are trying to avoid the bisphenol-A plastics.
FREEZING ICE
It expands
Water is such a basic thing, and it makes so many things easy, it seems like it ought to be simple through and through. But actually, a lot of thigns about the way it behaves are very unusual.
Most liquids, when they freeze, get heavier and sink. When water freezes, it expands and floats. This is because the molecules link up in a hexagonal form. This makes the molecules take up more space than when they're in a liquid state.
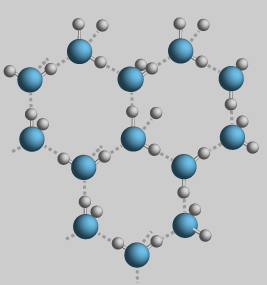
When water freezes, the water molecules link up in this open hexagonal shape. This is what makes ice expand as it freezes and what allows it to float.
(Diagram from Georgia State's HyperPhysics site)
The fact that ice expands when it melts is why icebergs float instead of sinking to the bottom of the sea, it's why ice forms on top of lakes and why we can go ice fishing, and this is why, when the water in the pipes in your house freeze, they will burst.

Glaciers. Brought to you by the fact that ice expands when it freezes.
(Photo from iOffer)
This is also why you don't want to fill an ice cube tray completely with water before freezing it: because the ice will expand above the separators in the tray and you'll have one great big block of ice rather than a bunch of separate cubes.
Does warm water freeze faster than cold?
It seems like a no-brainer, that if you start with cold water, it ought to freeze faster than warm water. Most of the time, in most of our home refrigerators, cold water will freeze faster than warm water. But in some cases, scientists have found, warm water may actually freeze faster than cold.
This is known as the Mpemba effect, after a Tanzanian high school student who first observed this phenomenon and whose experiments were described in a scientific magazine. (See, kids can be real scientists, too!) He demonstrated that it is possible for warmer water to freeze faster than cold water.
All sorts of variables affect the way in which water freezes: The temperature of the water, the temperature of the surrounding air, the temperature and size and shape of whatever is containing the water, whether the air over the water is moving and how fast, the humidity of the air, whether other impurities or gases are present in the water, and so on.
In fact, there are so many variables that affect the way in which water freeze, scientists can't account for every single variable in each instance and explain why water freezes exactly the way that it does each time it freezes. So they aren't entirely sure why it is that sometimes warmer water freezes faster than cold water. But they have some theories.
Possible reasons why warm water may freeze faster than cold include:
- In the warmer water, some of it has evaporated, so there is less water to cool and therefore it freezes faster.
- Cold water freezes from the top down. An ice sheet forms at the top which acts as a kind of insulator, protecting against further evaporation and slowing the cooling process. Warmer water doesn't get that ice sheet at the top and instead freezes throughout the liquid, creating something more like slush which freezes faster than water. This is what's known as a supercooled condition.
- There are fewer dissolved gases (bubbles) trapped in the warmer water. Since the warmer water molecules are moving around, it's easier for the dissolved gases (bubbles) to escape, so there are fewer of them. It's possible that the absence of bubbles means fewer obstacles for convection currents to bump into, so the colder temperature is transmitted more quickly and easily throughout the liquid, enabling it to freeze faster.
Clear ice cubes
You may have noticed that some restaurant ice cubes are a lot clearer and transparent than the ones you have at home. Why is that? And how can you make ice cubes that are just as clear and shiny?

These ice cubes are almost entirely transparent. No cloudiness. Just looking at them makes me thirsty.
(Photo from ifood)
OK, remember how all sorts of things affect the way ice freezes? Two of the factors that are important here are impurities and dissolved gases.
Impurities may be minerals present in the water (and which may be fairly important to your health, by the way) or they can be pretty much anything other than good old H2O. Dissolved gases, as we've learned, are bubbles trapped in with the H2O molecules. The presence of either one of these things can make the ice turn out looking cloudy.
So if you want to make really clear ice cubes, you want to reduce the number of impurities and dissolved gases. The best way to do reduce the impurities is to use distilled water. To let the dissolved gases escape before freezing the water, boil it first. (You might even wind up demonstrating the truth of the Mpemba effect besides.)
Ice makers also tend to produce ice that's less cloudy than ice made in trays because they use continually flowing water to assist in the freezing process, and the flowing water carries away the bubbles and some of the impurities, helping to keep the ice clear.
Ice spikes
Another thing that sometimes happens when you make ice cubes is they come out with little spikes on top. This doesn't happen very often, but every once in a while. Why is that? How come it doesn't happen more often?

Spikes on ice cubes
(Photo from Snowcrystals.com)
In most kitchen freezers with auto-defrosters, ice spikes won't usually appear. Or actually, they'll show up in a small way and only briefly before subsiding into smaller mounds.
Ice spikes show up and stay present in supercooled conditions. This means it's more like to happen in freezers that are especially cold, and if distilled water is used -- none of those impurities, again.
Ice spikes form when the water freezes quickly around the edges of the cube, leaving a small hole in the middle. As the ice expands during freezing, the unfrozen water gets pushed to that column in the middle and then it gets pushed up the hole above the surface of the water. Then, almost in the same way that an icicle freezes as water travels along it, that column of water freezes and the spike forms.
How an ice spike forms
(Diagram from Kenneth Librecht at CalTech)
MELTING ICE
More surface area melts faster
One of the truisms about melting is is that the greater the surface area of the ice cube, the faster the ice will melt. This means that a square ice cube will melt faster than a round one. This is why a lot of restaurants and hotels make round or spherically-shaped ice "cubes" -- so the ice will last longer and they won't have to make it as often.
Ice may melt faster in water
You might think that an ice cube on the counter top would melt faster than ice in your drink. If the air is significantly warmer than the liquid, this might be the case. But if they're about the same temperature, the ice in your beverage may actually melt faster.
The molecules in the liquid are more closely packed than those in the air. This means more molecules in the water are connecting with more of the surface area of the ice cube. The ice cube that has more surface area exposed will melt faster; therefore, the ice in the liquid will melt faster than the ice in the air.
Ice melts a lot at first
Actually, the ice on the countertop will not technically be "ice in the air" for long. The outer edges of an ice cube absorb heat first and the edges are where it melts first. It will seem to sit there in a little pool of liquid for a while. So that ice cube in the air will become partly an ice cube in liquid.
But it will seem to pause in that condition for much longer than it took to get to that partially melted state. that's because once some of the ice around the outer edges has melted, some of the energy has been used up in melting that ice. So the melting process will slow down once some of the ice has melted. The now-partial-cube will stay in that little pool of liquid until more heat arrives to melt more of the ice.
Ice melts from bottom to top
The fact that ice in water melts faster in air affects how ice on a lake melts. Even though the air above the ice on top of the lake may be warmer than the water below the ice, the ice on top of the water will melt from underneath first. This is especially the case if there's no snow covering the ice and sunlight is able to shine through the ice to the water below. That will really begin to warm the water, making the ice next to the water melt faster than the ice exposed to the air above.
As it melts, the ice will form "candles," sort of like icicles hanging down into the water. Because of the way they conduct light, they will make the ice look black. This is why darker-looking ice is actually less sturdy to walk on than ice that looks white or opaque.
Soon, melted ice water will slosh in between the candles and precipitate the melting process even further, causing the ice to break into chunks. Those chunks will have a greater surface area, and they'll melt even faster.
Sources
University of Florida, Gorrie's Fridge
Lloyd Copeman.com
Georgia State University, Department of Physics and Astronomy, HyperPhysics, The Expansion of Water Upon Freezing
University of California, Department of Physics, Can hot water freeze faster than cold water?
ThinkQuest, Cool Physics Phenomena, Hot Water Freezes Faster Then Cold
The Straight Dope, Which freezes faster, hot water or cold water?
TLC, How do you make clear ice cubes?
Kathryn Vercillo, HubPages, How To Make Clear Ice Cubes
Kenneth Librecht, CalTech, SnowCrystals.com
University of Toronto Department of Physics, Got Spikes on Your Cubes?
Jack Williams, USA Today, Water is strange when it freezes
UIUC, Ask the Van, Ice Cube Shape vs. Melting
DOE Ask A Scientist, How Does Ice Melt? February 22, 2004
Minnesota Climatology Working Group, How Lake Ice Melts
Mason Howard, eHow, Ice Cubes Melting Process
Came upon this by accident, fascinating!!!! Who knew?! Very Fun. I know I will reun!!
ReplyDelete